Utilizing renewable energy sources, such as wind and solar power, for water electrolysis to produce “green hydrogen” is a crucial pathway for promoting green electricity consumption, industrial decarbonization, and achieving carbon neutrality goals. Among existing technologies, alkaline water electrolysis (AWE), known for its technological maturity and low cost, is currently the mainstream method for green hydrogen production. However, when coupled with fluctuating renewable energy sources, its frequent start-stop cycles can induce a severe “reverse current” phenomenon. This leads to cathode oxidation and anode reduction, causing electrode corrosion and interface delamination, which severely constrain electrolysis efficiency and equipment lifespan. This dynamic stability challenge has become a core bottleneck limiting the large-scale and efficient coupling of alkaline electrolyzers with renewable energy.
Recently, the research team led by Professor Qiang Zhang and Associate Professor Cheng Tang from the Department of Chemical Engineering at Tsinghua University has achieved a breakthrough in the field of key materials for hydrogen production powered by fluctuating renewable energy. The team innovatively proposed a “gradient heterogeneous interface engineering” strategy, successfully developing a novel electrode material with excellent anti-reverse-current characteristics and industrial-grade electrolysis performance. This achievement provides a novel material solution for overcoming the key bottleneck of intermittent electrolysis using renewable energy, promising to propel the high-quality development of the green hydrogen industry.
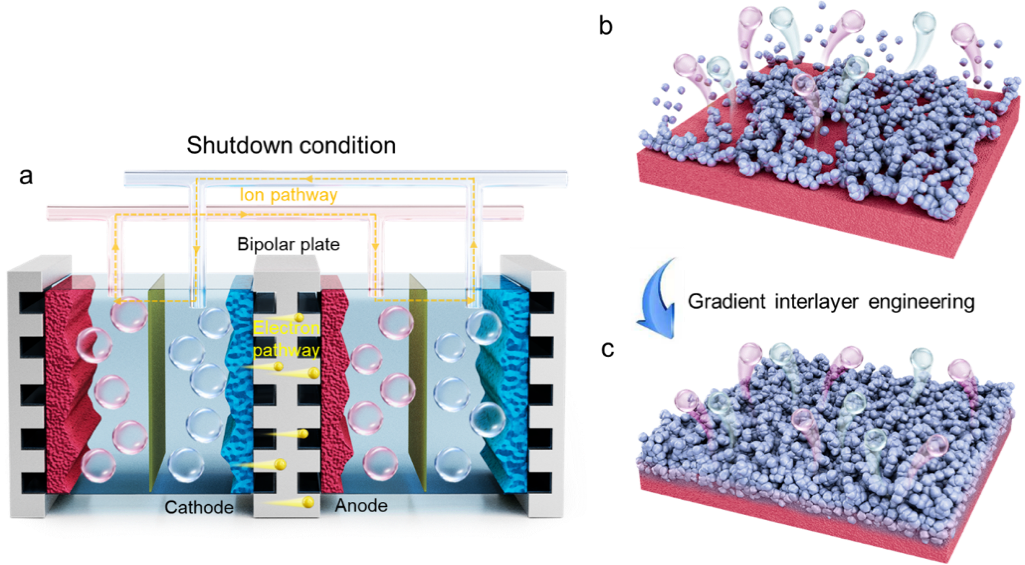
Figure 1. (a) Mechanism of “reverse current” generation after AWE shutdown. (b) Schematic of catalyst layer detachment and electrode corrosion during shutdown. (c) Schematic of the “gradient heterogeneous interface engineering” strategy and the resulting enhancement in electrode stability.
Diverging from traditional optimization of material composition and electronic structure, the research team focused on precise control of the interfacial crystallographic structure. Using a hot-injection process, they grew a Ni3S2 catalytic layer with a Ni/Ni3S2 gradient heterogeneous interface transition layer in situ on a commercial nickel mesh substrate. This structure effectively mitigates lattice mismatch and stress between heterogeneous materials through a “seamless interface”, promoting charge redistribution. This fundamentally enhances the mechanical and electrochemical stability of the interface under drastic potential reversal and high current density. Under industrial testing conditions (80°C, 30 wt% KOH), the electrode not only meets the U.S. Department of Energy’s 2026 activity target for alkaline electrolysis (1000 mA cm−2 @ 1.79 V) but also demonstrates zero performance decay after 3600 rigorous start-stop cycles. This breakthrough shatters the long-standing trade-off between “activity and stability” in this field, showcasing tremendous potential for industrial application.
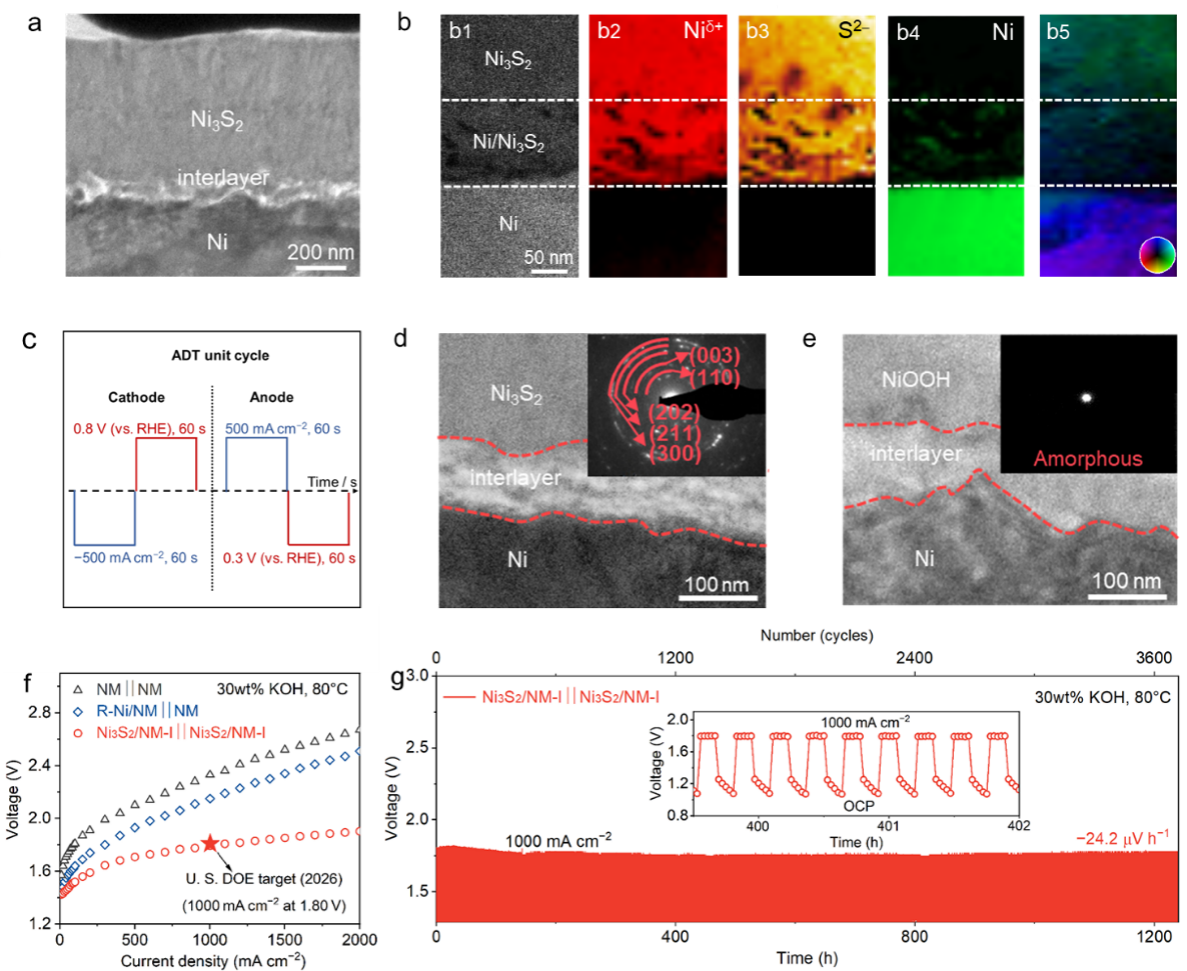
Figure 2. (a, b) Characterization of the electrode’s “substrate–transition layer–catalytic layer” interface. (c) Accelerated stress test simulating reverse current. Electrode interface characterization after 4000 cycles of accelerated stress tests acting as (d) cathode and (e) anode. (f) Electrode performance under industrial conditions. (g) Start-stop stability test of the electrode under industrial conditions.
The research findings have been published online on December 11 in the Journal of the American Chemical Society under the title “Heterointerface-Enabled Anti-Reverse-Current Electrodes for Alkaline Water Electrolyzers at 1000 mA cm−2”.
Professor Qiang Zhang and Associate Professor Cheng Tang are the co-corresponding authors of the paper. Postdoctoral researcher Wenjun He from Tsinghua University’s Department of Chemical Engineering, Postdoctoral researcher Yueshuai Wang from the School of Materials Science and Engineering at Beijing University of Technology, and Yilong Zhao from the School of Electrical and Electronic Engineering at Harbin University of Science and Technology are the co-first authors. The research received support from the National Key Research and Development Program of China, the National Natural Science Foundation of China, the China Huaneng Group’s Science and Technology Research Project, and Tsinghua University’s Independent Research Program.
Link to the paper: https://doi.org/10.1021/jacs.5c17603

