The chemical industry accounts for nearly 20% of China's industrial carbon emissions, and its low-carbon transformation urgently requires disruptive technologies. Under the "Dual Carbon" goals, "Green Electrification of Chemical Industry", which uses renewable energy to drive chemical production, has become a crucial development direction. Among these technologies, the electrosynthesis of hydrogen peroxide utilizes renewable electricity to directly convert water and air into hydrogen peroxide. This method offers advantages such as mild reaction conditions, strong compatibility with fluctuating green power, high product purity, and diverse application scenarios. It holds the potential to replace the traditional, energy- and waste-intensive anthraquinone process, serving as a model pathway for Green Electrification of Chemical Industry. However, the development of this technology has long been constrained by multiple challenges, including catalytic materials, electrode interfaces, and system integration.
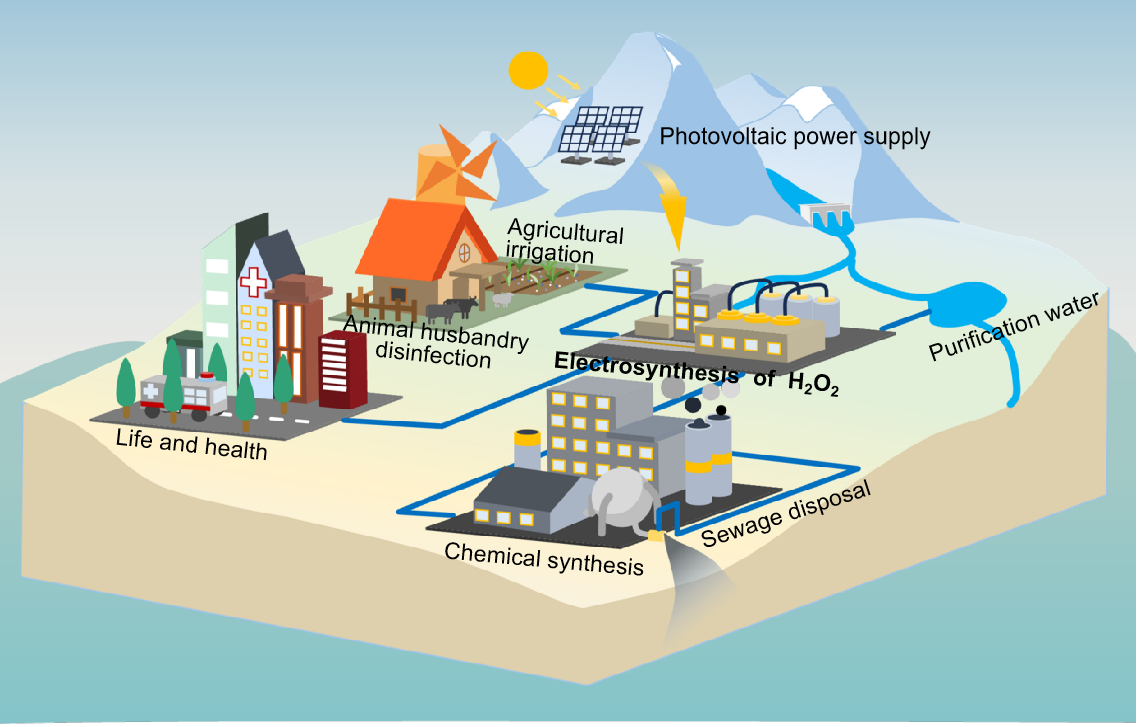
Figure 1. Distributed application scenarios for the green electrosynthesis of hydrogen peroxide.
To address these challenges, the team led by Professor Qiang Zhang and Associate Professor Cheng Tang from Department of Chemical Engineering, Tsinghua University, has conducted systematic research. They have developed a comprehensive technological framework spanning from atomic-level catalysts to reactors, achieving highly efficient, stable, and green-power-adaptable hydrogen peroxide electrosynthesis, laying the foundation for distributed Green Electrification of Chemical Industry.
At the catalyst level, the team created a cobalt single-atom catalyst with a unique oxygen-coordination structure. This catalyst maintains a selectivity for hydrogen peroxide exceeding 95% across a wide potential range. By employing nanoparticle-assisted dynamic replenishment of active sites, the catalyst demonstrated resilience through 55 start-stop cycles and stable operation for over 500 hours at a current density of –50 mA cm⁻2.
At the electrode interface level, the team designed a three-dimensional hydrophobic mesh gas diffusion electrode. By precisely regulating gas-liquid two-phase flow, they overcame the mass transfer bottleneck for reactant delivery and product removal in a zero-gap membrane reactor. This enabled the electrosynthesis of high-purity hydrogen peroxide using only pure water and air as feedstocks, without any electrolyte additives.
Building on these breakthroughs, the team developed an efficient and flexible green electrosynthesis system. This system allows for flexible adjustment of hydrogen peroxide concentration (153.6–2443.7 mg L⁻¹) and production rate (31.1–1.1 mL min⁻¹). It also maintained stable operation under simulated photovoltaic power fluctuation conditions, demonstrating high compatibility with renewable energy sources. This provides a viable technological pathway for applications such as distributed manufacturing, paper bleaching, smart agriculture, and wastewater treatment.
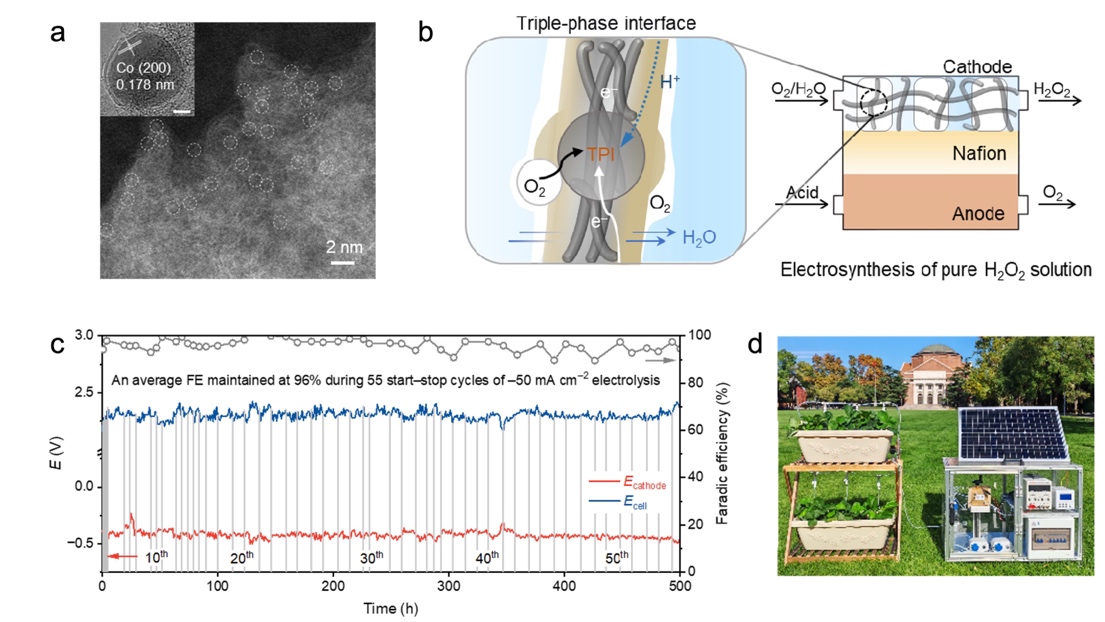
Figure 2. (a) Oxygen-coordinated cobalt single-atom catalyst. (b) Triple-phase interface engineering strategy for pure hydrogen peroxide electrosynthesis. (c) 500-hour stability test. (d) Demonstration of the green electrosynthesis system for smart agriculture.
The related research findings have been published in the journal Angewandte Chemie International Edition under the titles "Highly Stable Electrosynthesis of Hydrogen Peroxide Adapted to Fluctuating Renewable Energy" and "Electrolyte-Free Electrosynthesis of Pure H2O2 via Triple-Phase Interface Engineering."
Postdoctoral researcher Xinxin Li is the first author of the papers. Professor Qiang Zhang and Associate Professor Cheng Tang are the corresponding authors. The research was supported by the National Key Research and Development Program, the National Natural Science Foundation of China, the China Postdoctoral Science Foundation, the Shuimu Tsinghua Scholar Program, and Huaneng Group Science and Technology Research Project.

